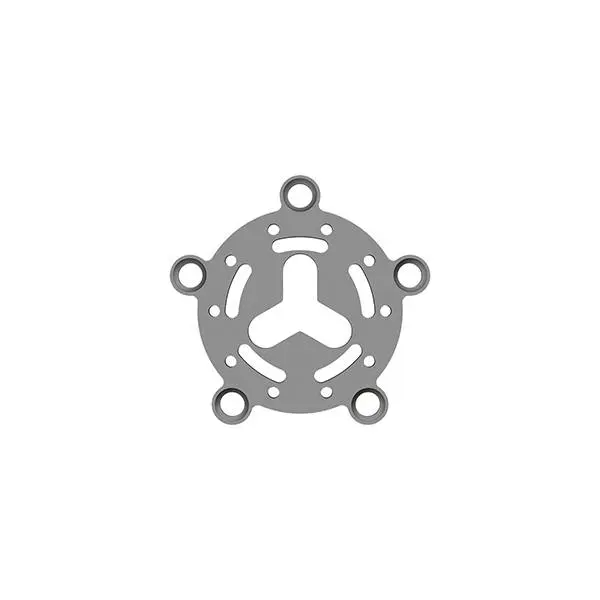
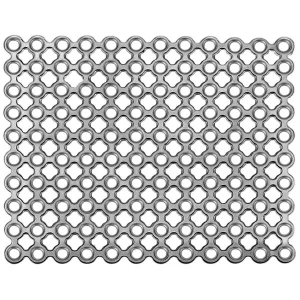
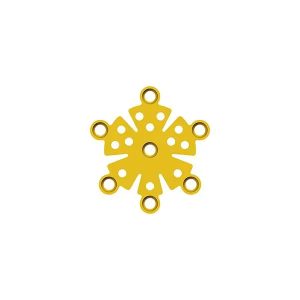
Description
Material: medical pure titanium
Product Specification
| Thickness | Item No. | Specification | ||
|---|---|---|---|---|
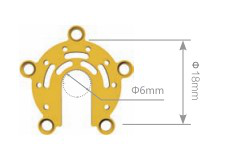 |
||||
| 0.4mm | 12.41.4010.181804 | Non-anodized | ||
| 12.41.4110.181804 | Anodized | |||
| 0.6mm | 12.41.4010.181806 | Non-anodized | ||
| 12.41.4110.181806 | Anodized | |||
| Thickness | Item No. | Specification | ||
|---|---|---|---|---|
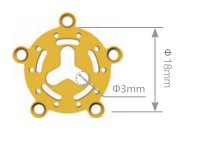 |
||||
| 0.4mm | 12.42.4010.181804 | Non-anodized | ||
| 12.42.4110.181804 | Anodized | |||
| 0.6mm | 12.42.4010.181806 | Non-anodized | ||
| 12.42.4110.181806 | Anodized | |||
| Thickness | Item No. | Specification | ||
|---|---|---|---|---|
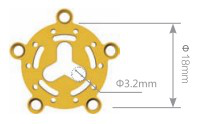 |
||||
| 0.4mm | 12.43.4010.181804 | Non-anodized | ||
| 12.43.4110.181804 | Anodized | |||
| 0.6mm | 12.43.4010.181806 | Non-anodized | ||
| 12.43.4110.181806 | Anodized | |||
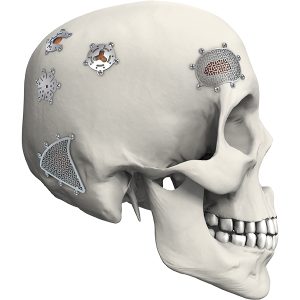
Features & Benefits:
• No iron atom, no magnetization in magnetic field. No effect to ×-ray, CT and MRI after operation.
• Stable chemical properties, excellent biocompatibility and corrosion resistance.
• Light and high hardness. Sustained protect brain issue.
• Fibroblast can grow into the mesh holes after operation, to make the titanium mesh and tissue integrated. Ideal intracranial repair material!
Matching screw:
φ1.5mm self-drilling screw
φ2.0mm self-drilling screw
Matching instrument:
cross head screw driver: SW0.5*2.8*75mm
straight quick coupling handle
cable cutter (mesh scissors)
mesh moulding pliers






Reviews
There are no reviews yet.