Description
What is 5.5 Minimally Invasive Pedical Screw System?
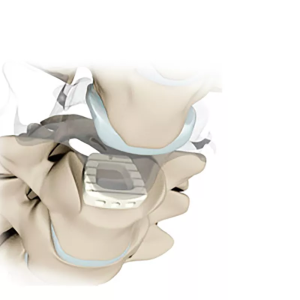
The 5.5 Minimally Invasive Pedicle Screw System is a medical device used in spinal surgery to provide stabilization and support to the spine. It is a type of pedicle screw system that is designed to be used in minimally invasive surgery (MIS) techniques, which involve smaller incisions and less disruption to the surrounding tissues compared to traditional open surgery.
The 5.5 Minimally Invasive Pedicle Screw System is typically made of titanium, a biocompatible metal that is strong, lightweight, and able to integrate with bone. The system consists of screws that are inserted into the pedicles (small bony projections on the back of the vertebrae) of the spine, and rods that are used to connect the screws and stabilize the spine.
The 5.5 Minimally Invasive Pedicle Screw System is available in different sizes and configurations to accommodate the specific needs of each patient and each surgical procedure. It is often used to treat a variety of spinal conditions, including degenerative disc disease, spinal stenosis, scoliosis, and spinal fractures.
What is the material of 5.5 Minimally Invasive Pedical Screw System?
The material used in 5.5 Minimally Invasive Pedicle Screw System can vary by manufacturer and product line. However, typically, the system is made up of titanium alloy or surgical-grade stainless steel. These materials provide strength, stability, and biocompatibility required for use in spinal surgery.
What are the types of 5.5 Minimally Invasive Pedical Screw System?
The 5.5 Minimally Invasive Pedicle Screw System is a type of spinal implant system that includes a variety of screws, rods, and other components designed for minimally invasive spine surgery.
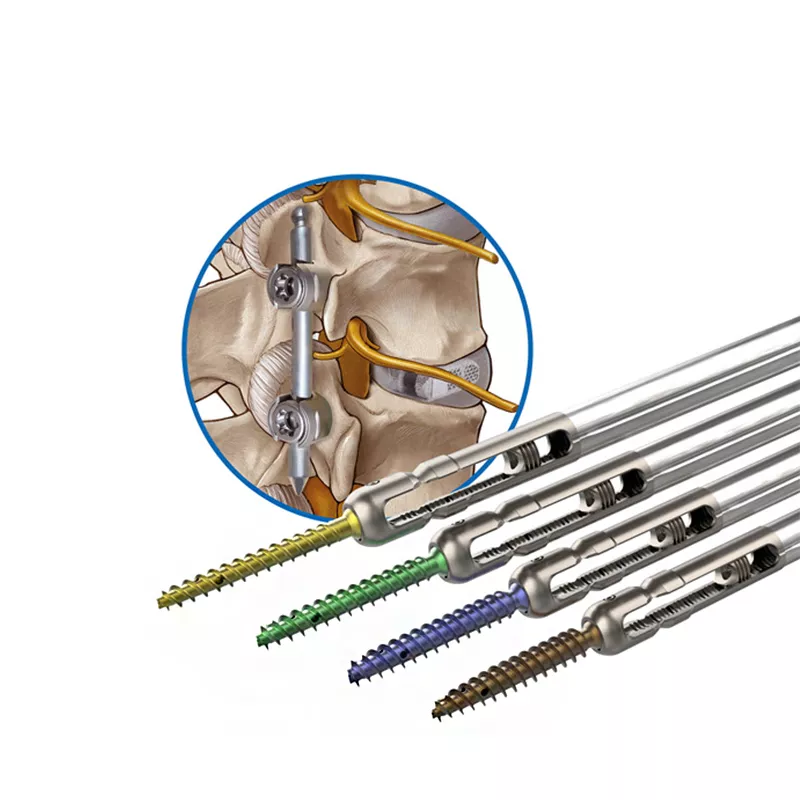
The specific types of screws included in the system can vary depending on the manufacturer, but generally include polyaxial screws, uniplanar screws, and cannulated screws. These screws can be used with different types of rods, such as straight or pre-bent, to achieve the desired spinal alignment and stability.
Some 5.5 Minimally Invasive Pedicle Screw Systems also include specialized instrumentation to aid in the implantation of the screws and rods.
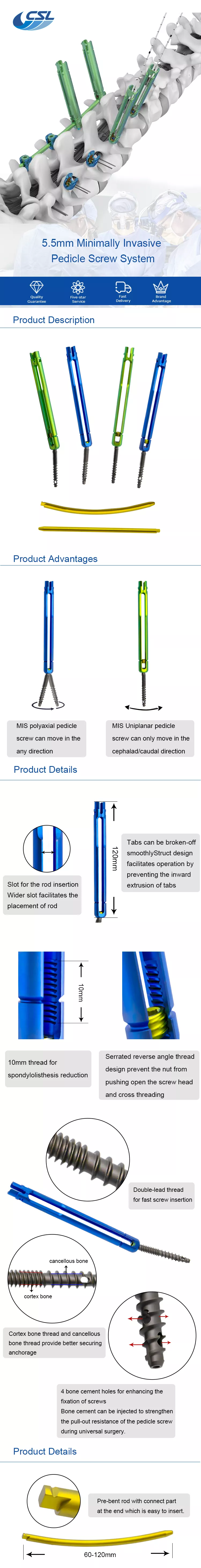

How to use 5.5 Minimally Invasive Pedical Screw System?
The 5.5 Minimally Invasive Pedicle Screw System is a surgical instrument used to provide stabilization to the spine during spinal fusion surgery. Here are general steps on how to use the system:
- Preoperative planning: The surgeon will assess the patient’s medical history and imaging studies to determine the appropriate size and type of screws to be used in the procedure.
- Anesthesia: The patient will be placed under general anesthesia to ensure comfort and safety during the procedure.
- Incision: A small incision will be made over the affected area of the spine to allow access to the vertebral bodies.
- Pedicle preparation: The pedicle of the vertebra will be prepared for screw placement using specialized tools such as awls and taps.
- Screw placement: The 5.5 Minimally Invasive Pedicle Screw System will be used to insert the screws into the prepared pedicles. Fluoroscopy or navigation systems may be used to ensure accurate placement.
- Rod placement: The screws will be connected by a rod, which will provide stability to the spine.
- Wound closure: The incision will be closed using sutures or staples.
The exact technique used may vary depending on the specific patient and surgeon. It is important to follow the instructions provided by the manufacturer and to receive proper training before using the system.
What are 5.5 Minimally Invasive Pedical Screw System used for?
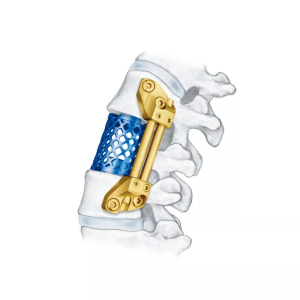
The 5.5 Minimally Invasive Pedical Screw System is used in spinal surgeries to provide stabilization and correction for spinal disorders such as degenerative disc disease, spinal fractures, scoliosis, and other conditions.
This system is designed to provide a minimally invasive approach to spinal surgery, which can lead to faster recovery times and reduced blood loss and tissue damage compared to traditional open surgeries.
The screws are inserted into the pedicles of the vertebrae and connected by rods to stabilize the spine and correct any deformities. This system is often used in conjunction with other spinal implants and instruments, depending on the specific needs of the patient.
How to Buy High Quality 5.5 Minimally Invasive Pedical Screw System?
To buy a high quality 5.5 minimally invasive pedicle screw system, follow these steps:
- Identify reputable manufacturers and suppliers: Look for manufacturers and suppliers with a good reputation in the market. You can search online, ask for referrals from your colleagues, or attend medical conferences to identify potential suppliers.
- Check for certifications: Ensure that the manufacturer has obtained relevant certifications such as ISO 13485, which indicates compliance with medical device manufacturing standards.
- Consider the quality of the material: The 5.5 minimally invasive pedicle screw system should be made of high-quality materials such as titanium, which is strong, durable, and compatible with the human body.
- Check for FDA approval: Ensure that the system has been approved by the FDA, which guarantees safety and effectiveness.
- Check for customer reviews: Check for customer reviews and ratings of the system to gauge its performance and reliability.
- Consider the cost: Compare the prices of different manufacturers to find the best quality system at an affordable price.
- Check for after-sales services: Ensure that the manufacturer provides after-sales services such as training, technical support, and warranty for the system.
By following these steps, you can buy a high quality 5.5 minimally invasive pedicle screw system that meets your requirements and delivers excellent results.








Reviews
There are no reviews yet.